
Hopefully you found this helpful and thank you for watching. Another thing about a mixture is it does not have a definite formula. It does not look the same throughout the entire salad. If you're looking at iron ore you can see different parts. So that leaves the fact that it is a mixture really and typically it's a heterogeneous mixture because you can see definite different parts in it. So that tells us it cannot be a compound nor can it be an element. Part 2: Classify each of the following as elements (E), compounds (C) or Mixtures (M). Um So what we find is there is not you're not going to find a definite formula for iron ore. The makeup of iron ore is going to vary from one location to another, just based upon the ground.
Classify the following as element compound or mixture plus#
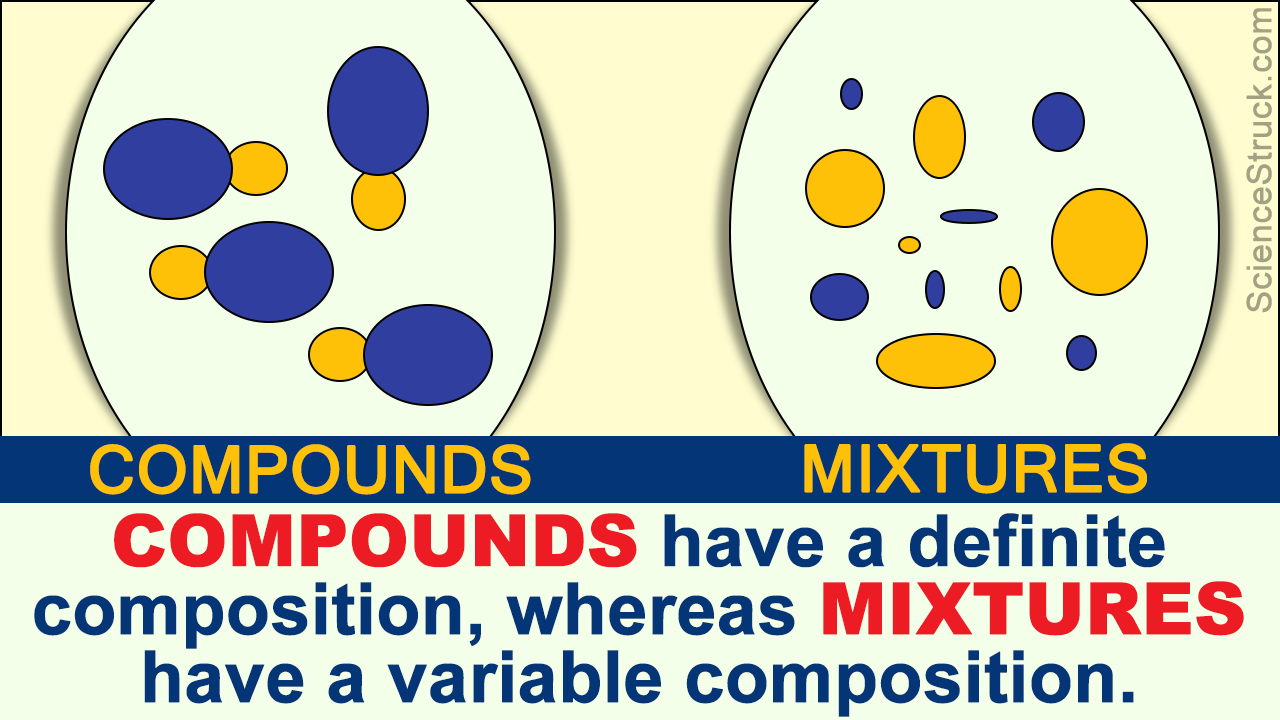
Well iron ore is composed usually of iron oxides which would be compounds but plus minerals and the makeup of iron war. Okay, so this question wants to know if iron ore is a mixture compound or an element. Okay, mixtures could be broken down further into heterogeneous mixtures where we can see visibly different parts or homogeneous mixtures or homogeneous mixtures which are uniform throughout like salt dissolved in water. If the answer is no about separated by physical means, we have a pure substance and a pure substance can be further broken down into compound or element compounds have a specific formula like N. Another thing about a mixture is it does not have a definite formula. Answer to Classify each of the following as an element, a compound.

If the answer to that is yes then that means what we had was a mixture.

We can ask ourselves if it can be separated by physical means. When we look at a sample of matter, we can ask ourselves a question. I want to talk about a breakdown of matter.


 0 kommentar(er)
0 kommentar(er)
